From enthalpy to industrial processes, from hydrocarbons to titrations; the HSC Chemistry course no doubt spreads across a myriad of different areas which can be daunting at first for any student. However, knowing your chemical reactions and processes is undeniably the key to conquering HSC Chemistry, and will sufficiently prepare you for every challenge that comes your way. And so, here is a comprehensive guide to all the chemical reactions and processes you should know by your HSC Chemistry exam to secure that Band 6 you deserve 😊
HSC Chemistry Module 5: Equilibrium Reactions
Analysing Reversibility
Module 5 centres on the concept that reactions can be reversible. The following are reactions, commonly taught through a practical, that the syllabus requires you to note observations for and analyse their reversibility: i.e. justify why they are either reversible or non-reversible.
Reversible:
- Cobalt(II) chloride hydrated and dehydrated
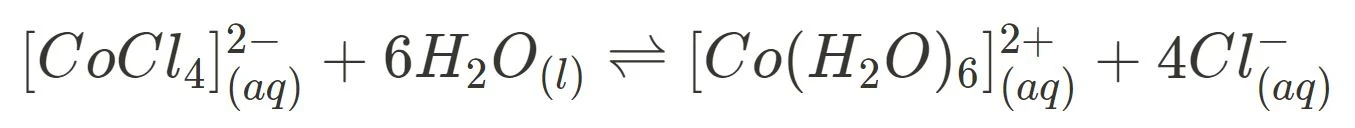
Cobalt(II) chloride can turn from blue to pink when hydrated and vice versa when dehydrated. The reversibility in colour, thus indicates the reversibility of the reaction.
- Iron(III) nitrate and potassium thiocyanate
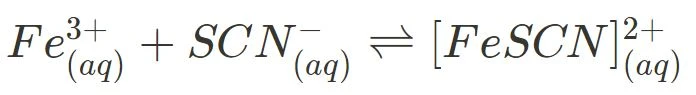
Increasing or decreasing the concentration of reactant or product leads to a visible shift in colour. More specifically, iron(III) ions are orange-yellow, thiocyanate ions are colourless and iron-thiocyanate complexes are blood red in solution. These changes in colour represent the reaction’s ability to go in specific directions, both the forward and reverse.
Non-Reversible:
- Burning Magnesium
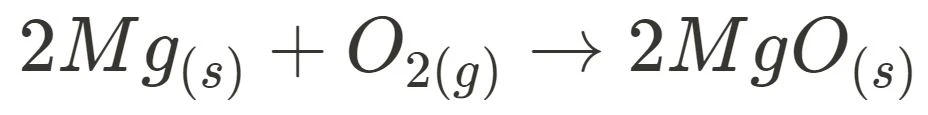
- Burning Steel Wool
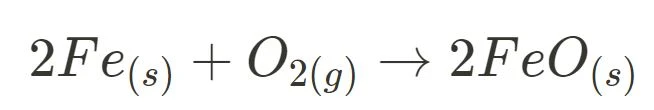
For both burning magnesium and steel wool, no reactant is reformed as only the forward reaction occurs. Thus, they can be concluded as irreversible reactions.
Non-equilibrium Systems and Enthalpy, Entropy and Gibbs Free Energy
Two specific non-equilibrium reactions are combustion (such as the combustion of glucose, i.e. cellular respiration):
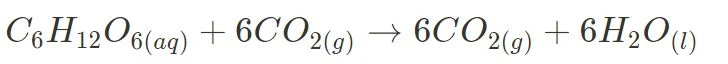
and photosynthesis.
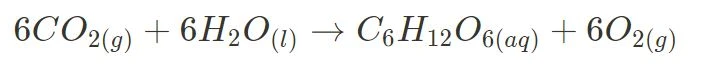
For these two reactions, the syllabus expects you to explain their irreversibility by analysing their change in enthalpy, entropy and Gibbs Free Energy. Although exact values are not compulsory to memorise, you should understand how the large magnitude of their change in Gibbs Free Energy and other aspects unique to the reaction (such as photosynthesis being a multi-step reaction) contribute to their irreversibility.
Applications of Le Chatelier’s Principle
The following are prescribed reversible reactions that are very, VERY commonly included in exams to assess your ability of applying Le Chatelier’s Principle. Each involves a particular disturbance, such as the reaction between nitrogen dioxide and dinitrogen tetroxide being impacted by a change in volume/pressure. However, they can be recontextualised to involve a different change, and thus it is recommended to prepare yourself such that you can justify all changes in relation to LCP.
- heating cobalt(II) chloride hydrate → temperature
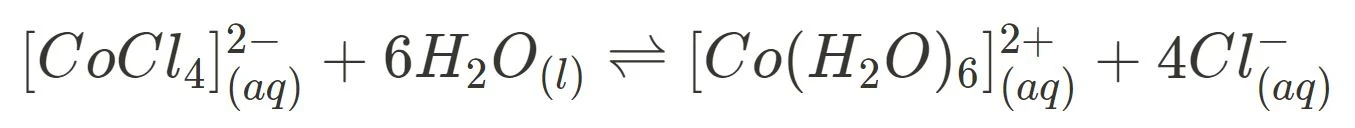
- interaction between nitrogen dioxide and dinitrogen tetroxide → volume/pressure
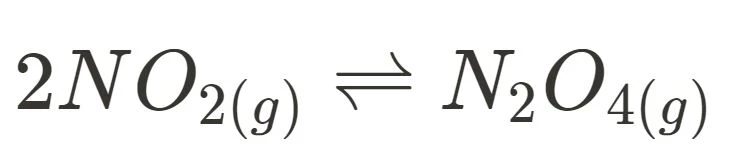
- iron(III) thiocyanate and varying concentration of ions → concentration
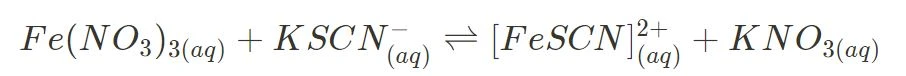
Solubility Equilibria
This section requires you to be familiar with two different areas: the dissolution of toxins in cycad fruits, which can be sufficiently expressed through
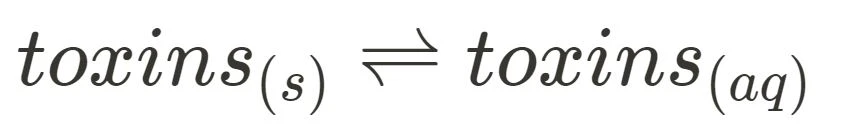
(or another equation approved by your school), and applications of solubility rules to precipitation reactions. In regards to the latter, although the syllabus lists three certain reactions, exams tend to not only test you on those specific cases but can introduce other unfamiliar salts and ions. To combat this, make sure to ground your understanding of solubility rules and practise applying them to a wide range of substances.
HSC Chemistry Module 6: Acid Base Reactions
General Reactions Involving Acids
In Module 6, the following are general chemical reactions involving acids that are listed in the syllabus and should already have been taught to you.
- Acid + Base
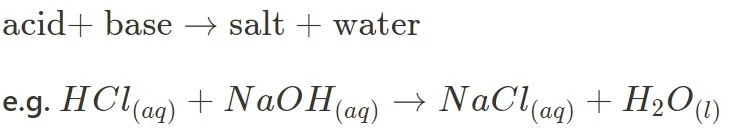
- Acid + Carbonate
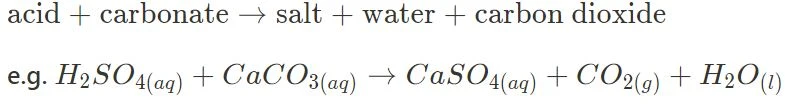
The above in particular are essential in this module, and further content is built on this knowledge. Make sure you are able to identify those reactions, and comfortably predict their products.
Some more useful reactions to take note of include:
- Acid + Metal
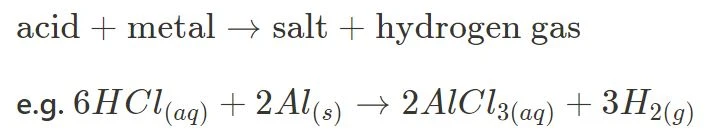
- Acid + Metal Oxide
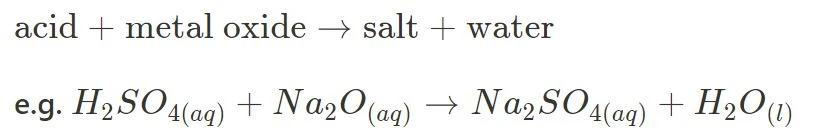
- Acid + Ammonia
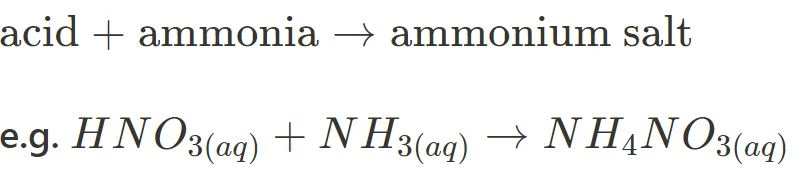
If you’re looking for tips to do better in Module 6, read this article!
Acid and Base Strength and Ionisation
In this module, you will be introduced to the concept of acid and base strength. There are a couple of examples of strong acids and bases, as well as weak acids and bases that NESA expects you to know. For those, you will often be required to write their ionisation equations, and explain why we use complete arrows for strong acid and base ionisation, and reversible arrows for weak acids and bases.
Below are the general ionisation equations for strong and weak acids, and strong and weak bases:
- Strong Acid Ionisation
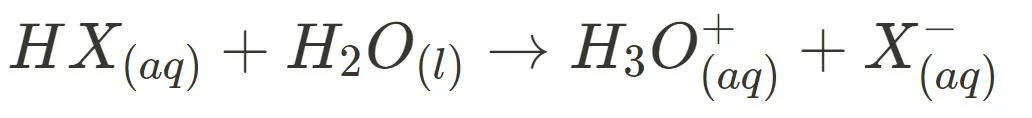
- Weak Acid Ionisation
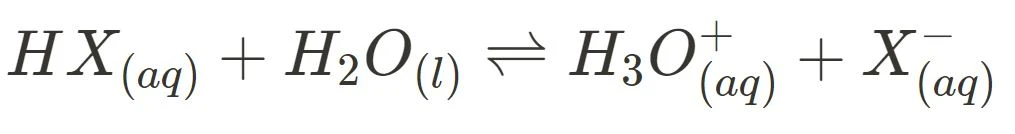
- Strong Base Dissociation
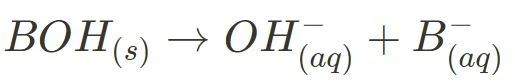
- Weak Base Ionisation
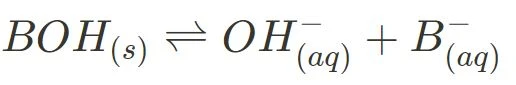
Please note, you can be marked down for using the incorrect arrow in your equations, and it is crucial to be able to identify the strong and weak substances for further content (e.g Ka, or the acid dissociation constant).
Amphiprotic Substances
As you may know by now, amphiprotic substances are substances that can act as a Bronsted-Lowry acid or base, depending on conditions. The syllabus requires you to be able to write ionisation/dissociation equations that show the amphiprotic nature of two specific ions:
- Sodium Hydrogen Carbonate
Hydrogen carbonate is the first amphiprotic ion you need to know. As the conjugate base of carbonic acid, as well as the conjugate acid of carbonate, hydrogen carbonate can both donate or accept protons depending on conditions.
To demonstrate this, you need two equations:
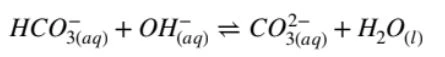
where hydrogen carbonate acts as an acid in basic conditions, and:
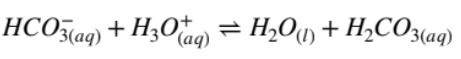
where hydrogen carbonate acts as a base in acidic conditions (evident through proton transfers).
- Potassium Dihydrogen Phosphate
Similarly, you should be able to show the amphiprotic nature of the dihydrogen phosphate ion via the following equations:
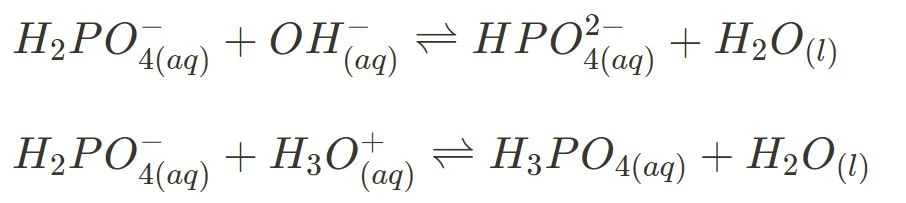
where it acts as an acid in basic conditions, and:
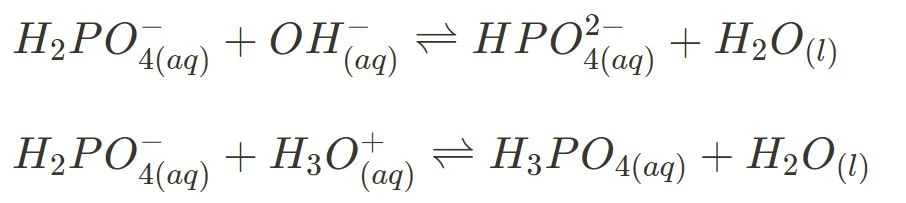
where it acts as a base in acidic conditions.
Natural Buffer Systems
Buffer systems is another topic in Module 6, and you need to thoroughly understand HOW they can minimise small changes in pH. In particular, the last dot-point asks you to investigate naturally-occurring buffer systems and their importance. Here are two common and effective examples:
- Blood Buffer System
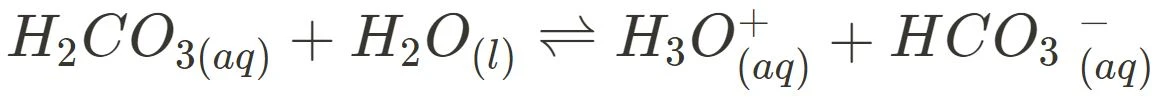
The carbonic acid-hydrogen carbonate buffer in blood allows the maintenance of the narrow pH range 7.3-7.4 to ensure the optimal functioning of enzymes and proteins in the body which can otherwise stop working or denature outside of this range.
- Natural Waterways Buffer System
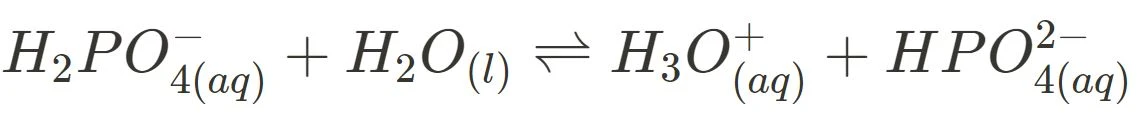
Several aquatic organisms can only survive in certain pH ranges and can be very sensitive to changes in pH via pollution. For example, coral reefs require a pH between 7.7 and 8.4 for a flourishing marine environment. Hence, the dihydrogen phosphate-hydrogen phosphate buffer is essential in ensuring these conditions are met.
For the above buffer systems, you need to understand and be able to explain how pH is maintained through equilibrium shifting and Le Chatelier’s Principle upon the addition of an acid and a base.
Need a hand writing balanced chemical equations? Read our Study Guide to Year 11 HSC Chemistry Module 2 - Introduction to Quantitative Chemistry to get back up to speed!
HSC Chemistry Module 7: Organic Chemistry
As the name suggests, Module 7 introduces you to a new family of substances - organic compounds. Whilst you may have heard some substances prior, this section of the syllabus can be particularly challenging as it requires you to memorise and predict products for a bulk of unfamiliar reactions unique to organic compounds. But fear not! With practice and the list below, you can systematically memorise each and every one!
Addition Reactions
Addition reactions occur when there is an addition of a small molecule to the double or triple bond of unsaturated molecules (alkenes and alkynes). There are four key addition reactions you will be required to recognise and apply:
Hydrogenation:
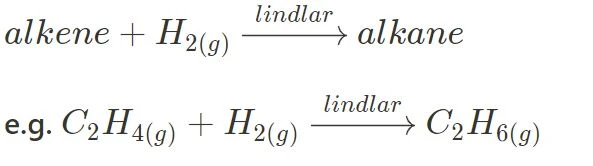
This reaction involves the addition of two hydrogen atoms to the alkene molecule, and uses the Lindlar (palladium) catalyst.
Hydration
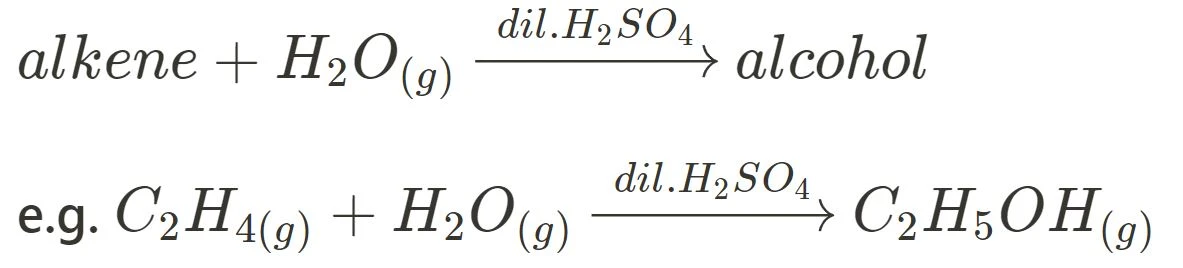
Hydration refers to the reaction between an unsaturated alkene and water to produce an alcohol. Typically, diluted sulfuric or phosphoric acid is utilised as a catalyst.
Halogenation
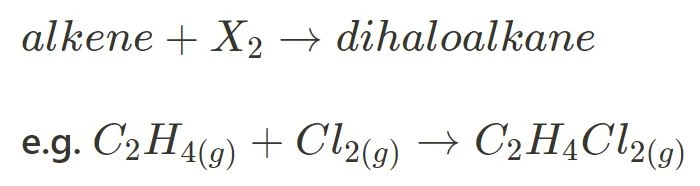
As the name suggests, halogenation involves the addition of atoms from Group 7, the halogens, to an alkene molecule to form a dihaloalkane (an alkane with two halogen atoms attached to adjacent carbon atoms). Note that halogenation does not need a catalyst as halogens are naturally reactive enough to induce the reaction.
Hydrohalogenation
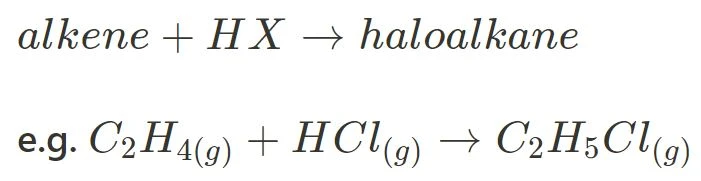
Hydrohalogenation is similar to halogenation in that it also does not require a catalyst and forms a haloalkane. However, the reagent is the key difference.
Something that is very important to consider is that addition reactions such as hydrohalogenation can form a major or minor product, based on Markovnikov’s rule. If this is something you find unfamiliar, I suggest you review Markovnikov’s rule and practice applying it to some addition reactions!
Substitution Reactions
A substitution reaction occurs when an atom or functional group in a saturated hydrocarbon molecule is replaced or ‘substituted’ by another atom or group to form two products. The halogenation of saturated alkanes is the most common substitution reaction you will be exposed to.
For example, the bromination of propane:
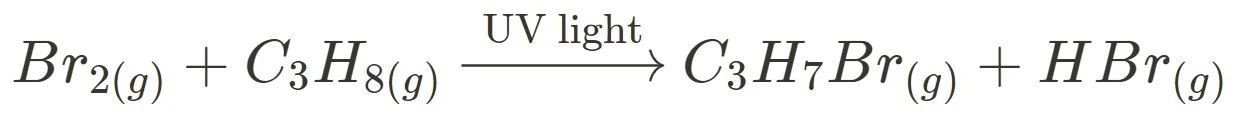
A C-H bond of propane is broken, allowing for a bromine atom to substitute the hydrogen to form the haloalkane. However, do note that substitution reactions tend not to occur spontaneously. This is due to the stability of C-H bonds, and the large input on energy required to break it. Hence, substitution reactions involve UV light as a catalyst such that the C-H bond can be broken and the substitution can take place.
Reactions with Alcohols
The syllabus expects students to be able to write equations, state conditions and catalysts, and predict products for the following reactions with alcohols as one of the reagents.
Combustion
The combustion of alcohol refers to the reaction between an alcohol and oxygen, and can be complete or incomplete.
- Complete:
For complete combustion, the products will always be carbon dioxide and water, as shown below.
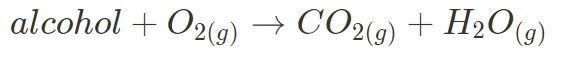
- Incomplete:
In incomplete combustion, products other than carbon dioxide and water, primarily carbon soot and carbon monoxide, are also formed. Keep in mind that the reaction below and its products are only one possibility.
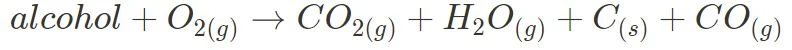
Dehydration
The dehydration of an alcohol involves the removal of a hydrogen atom and the hydroxyl group to form an alkene and a water molecule, like so:
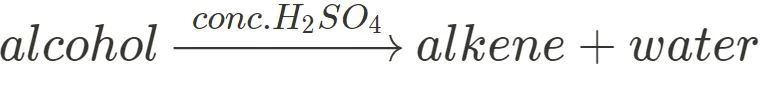
This reaction requires concentrated sulfuric acid or phosphoric acid as a catalyst.
Substitution with HX
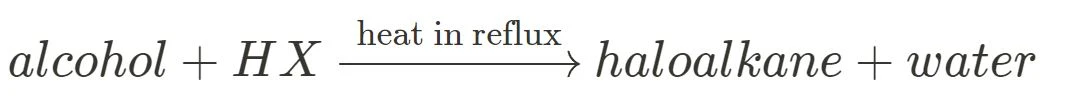
Upon the addition of HBr, HCl or HI, the hydroxyl group of alcohols can be substituted by a halogen to produce a haloalkane and water, as shown above. This reactions requires heating under reflux at low temperatures (20-30 degrees Celsius).
Oxidation of Alcohols
Oxidation refers to the loss of electrons in a chemical species, and can only occur in the presence of an oxidising agent, also known as an oxidant, that can take the electrons being lost. In this case with alcohols, they can be oxidised by acidified potassium permanganate or acidified potassium dichromate, and their products differ depending on whether they are a primary, secondary or tertiary alcohol.
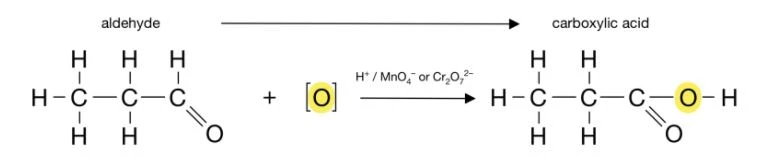
The complete oxidation of primary alcohols involves two steps: the conversion of the alcohol to an aldehyde, and then the conversion of the aldehyde to a carboxylic acid. For example:
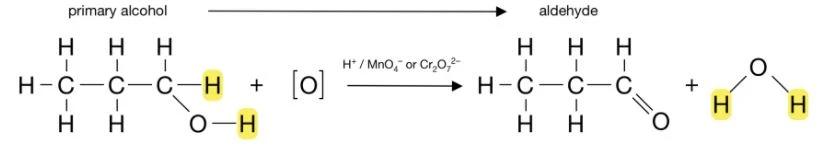
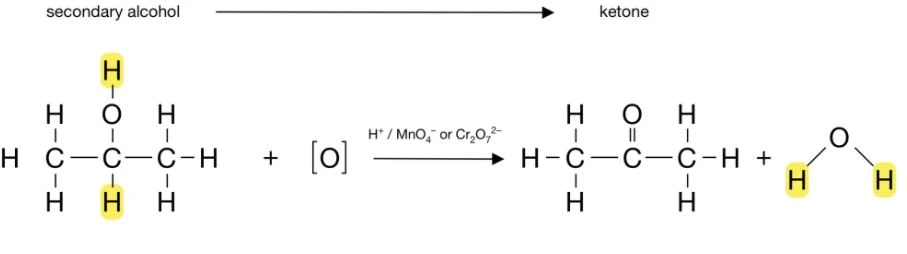
Secondary alcohols are only able to undergo oxidation once, which is the conversion of the alcohol to a ketone. For example:
Tertiary alcohols cannot undergo oxidation, hence if writing an equation the following example would be sufficient:
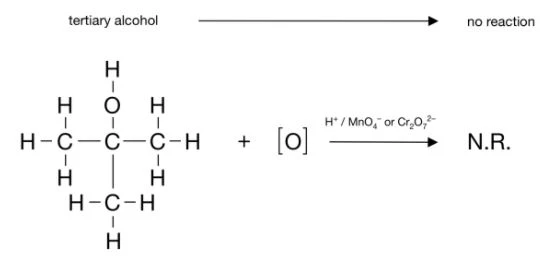
It is recommended that you review why this is in your own time if unsure why.
Production of Alcohol
As well as reactions involving alcohols, you will need to solidify your understanding on the ways alcohols can be produced.
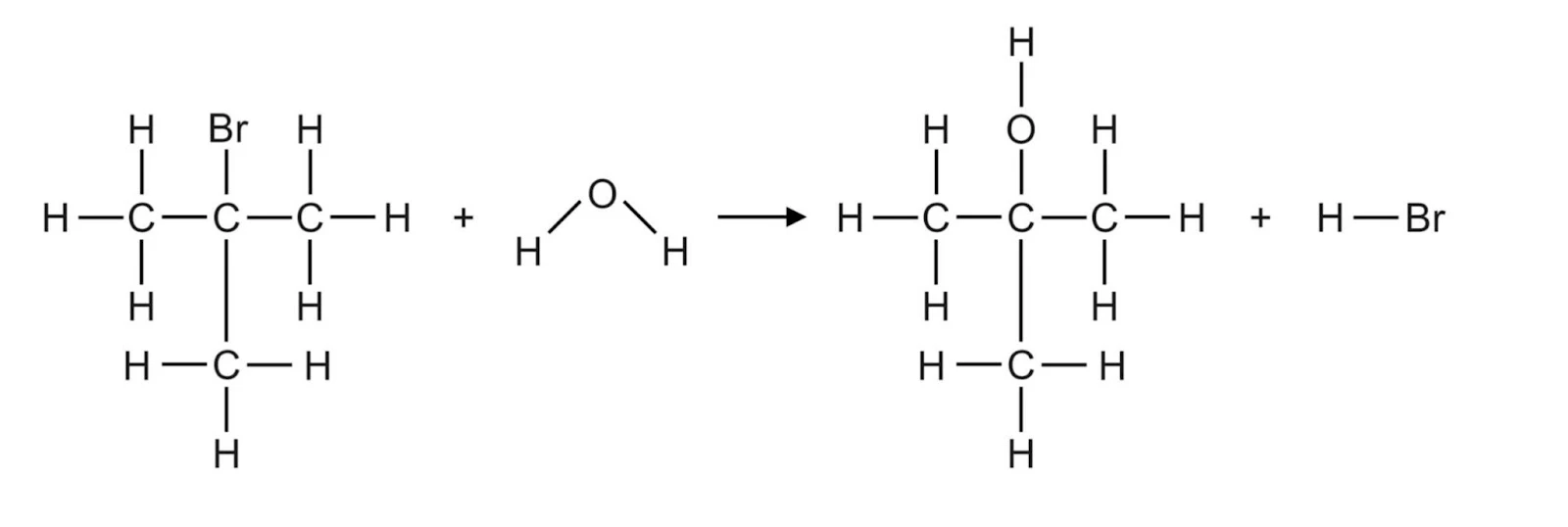
Substitution with Halogenated Organic Compounds
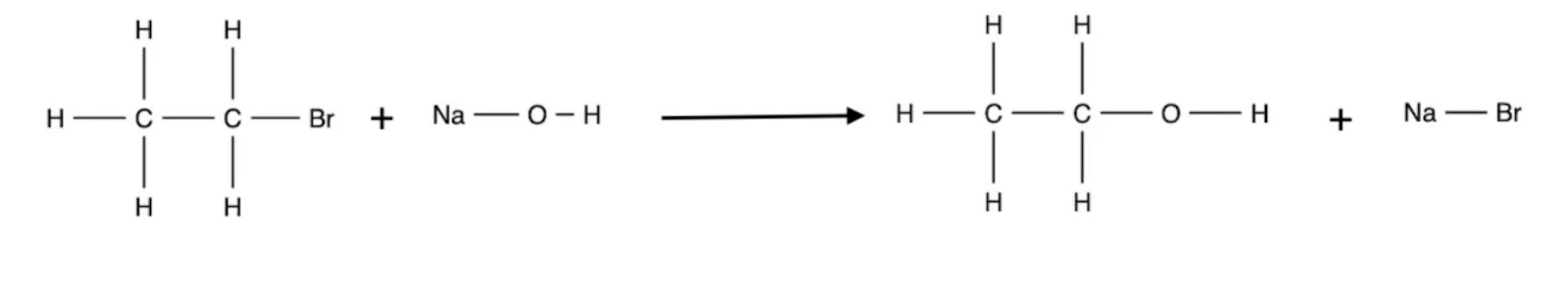
In this process, a hydroxyl group replaces the halide atom on the haloalkane to form an alcohol. This process is generally heated under reflux at low temperatures using sodium hydroxide or potassium hydroxide as reagents for primary and secondary alcohols, whilst water is generally utilised for tertiary alcohols.
- Primary:

- Secondary:

*same molecular equation as above, but note that the bromine atom is on a different carbon atom!*
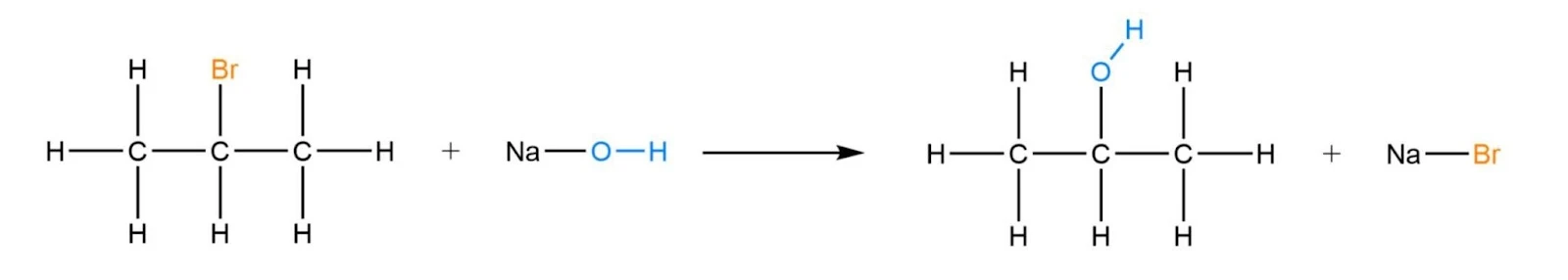
- Tertiary:
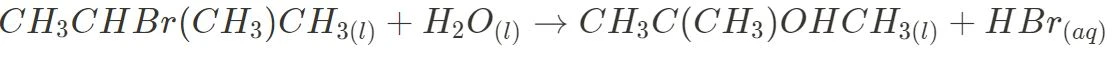
Fermentation of Glucose
The fermentation of glucose produces ethanol, and is as follows:
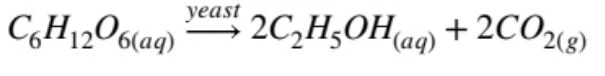
Fermentation can only occur in specific reaction conditions such as temperature, as well as the requirement of yeast as a catalyst. (If you haven’t revised these conditions make sure you go over them!)
It is also the key process in producing bioethanol, a sustainable fuel source compared to crude oil. Knowing the advantages and disadvantages of both fuel sources and using your knowledge on the reactions involving alcohols will be extremely helpful when answering extended response questions of this module.
Esters
Esterification, the production of esters, is an extremely important reaction that students look at in Module 7. The practical in particular should be fleshed out in your own time, however the general reaction for esterification is the following:
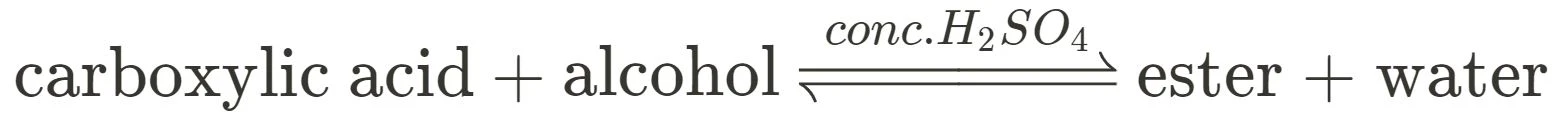
In regards to esterification, make sure to note that it is a reversible reaction (always use equilibrium arrows!) and that concentrated sulfuric acid is utilised as a catalyst.
Polymers
In exams or in class, you may be asked to write a structural equation representing the polymerisation of an addition or condensation polymer. The way in which we do this differs from our conventional structural equations, and this can be seen through the use of different brackets in the following:
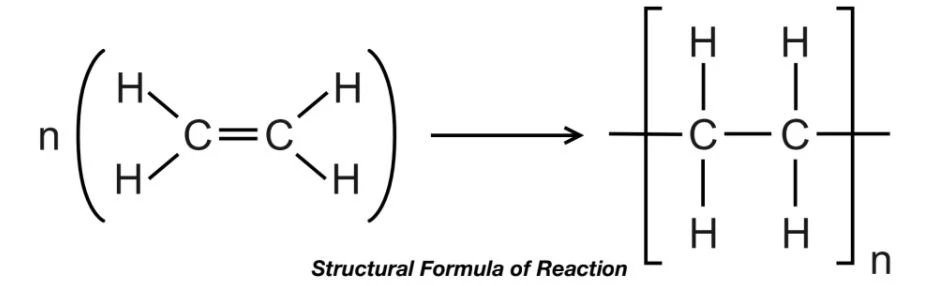
Addition Polymers
- Polyethylene
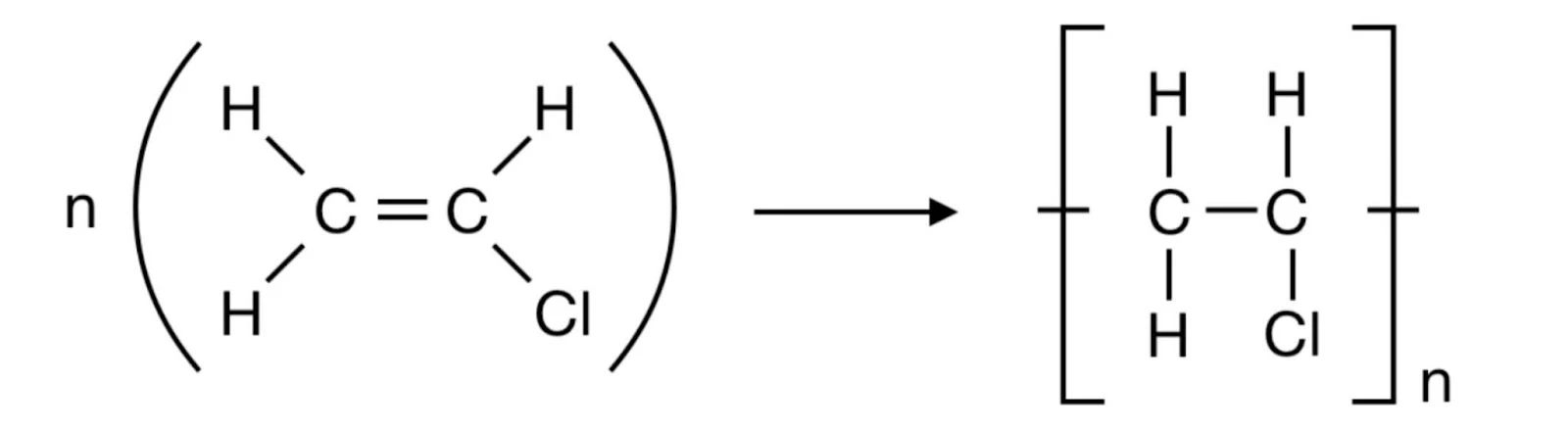
- Polyvinylchloride
- Polystyrene
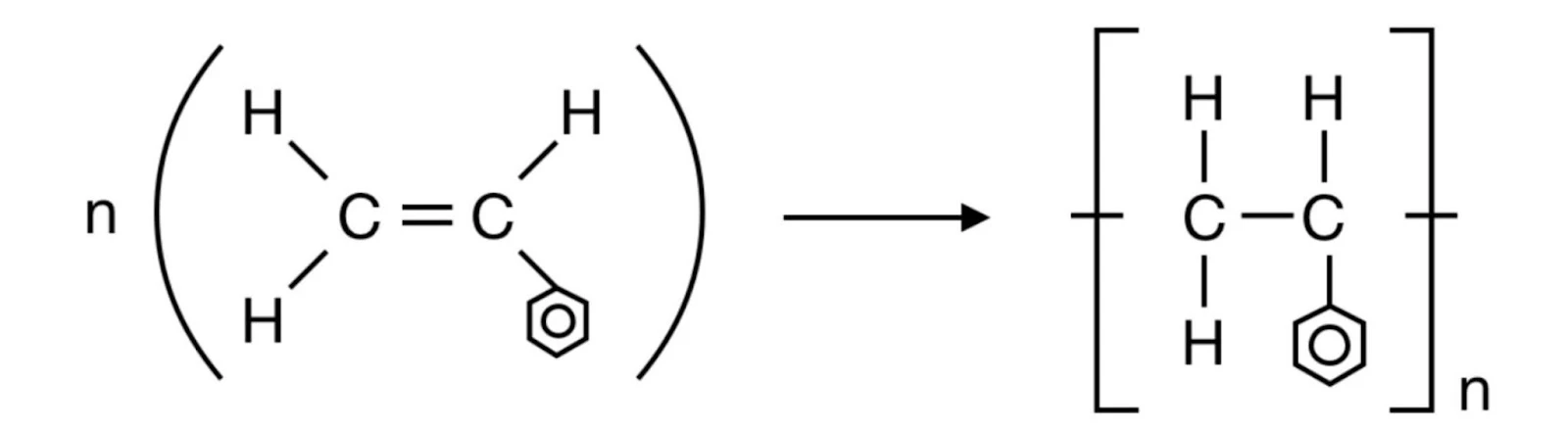
*The hexagon and circle is the condensed structural formulae of a benzene ring*
- Polytetrafluoroethylene (or Teflon)
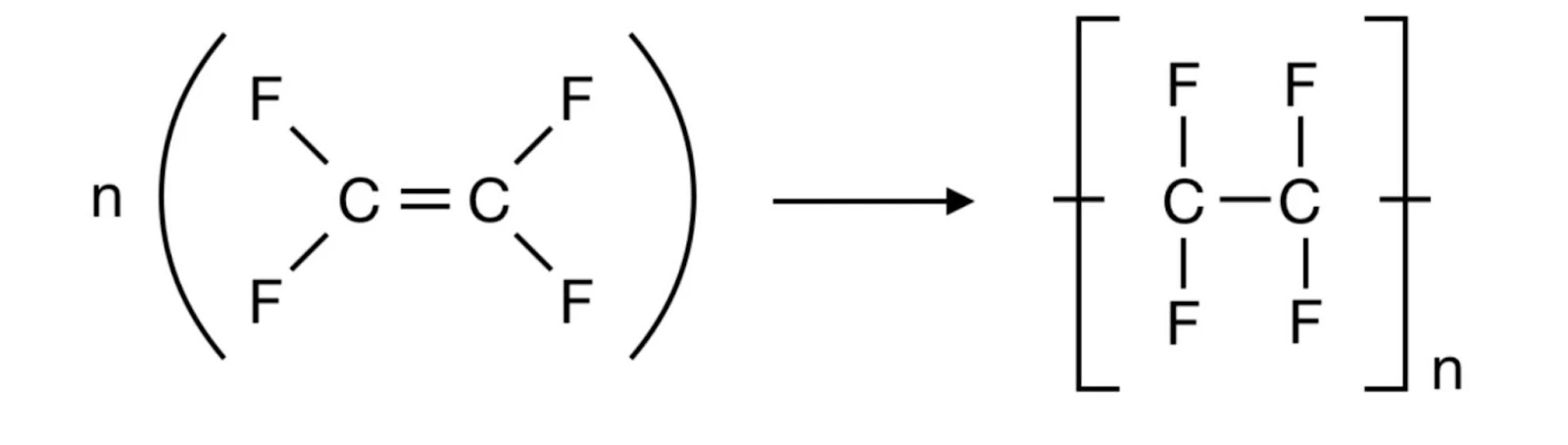
Condensation Polymers
- Polyester
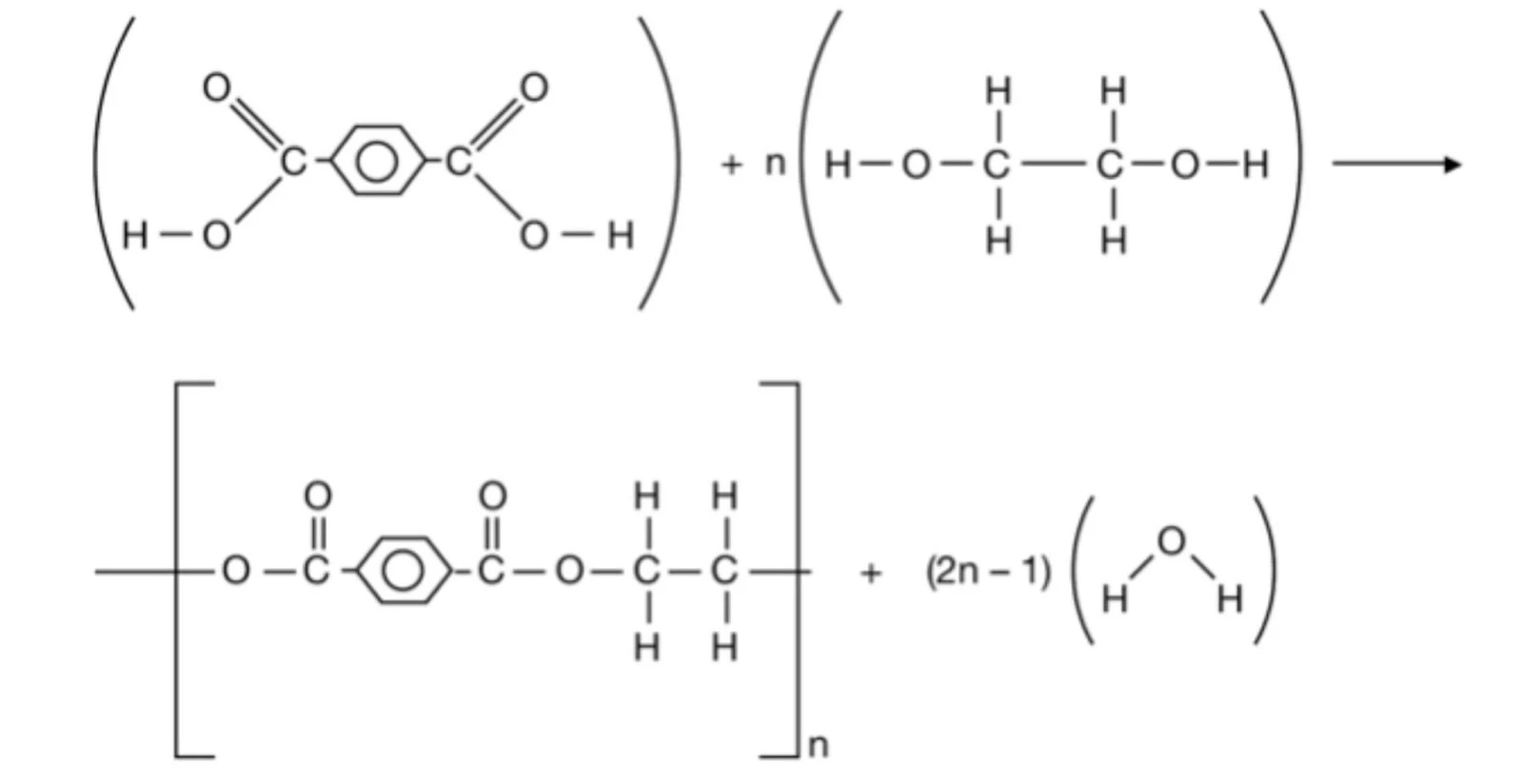
- Nylon-6
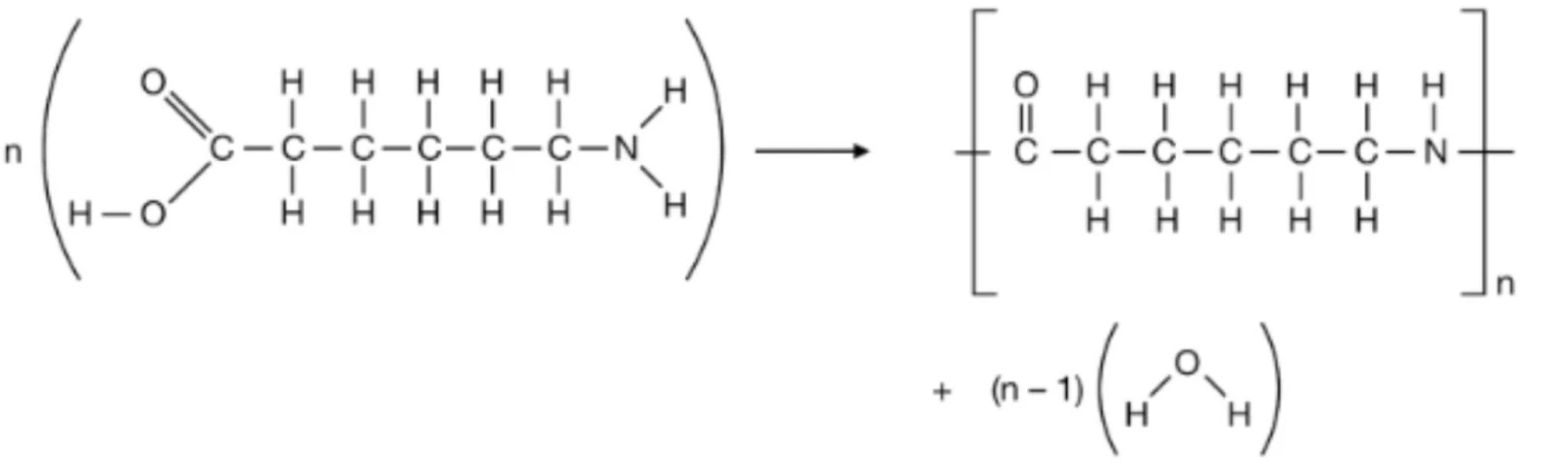
- Nylon-6,6
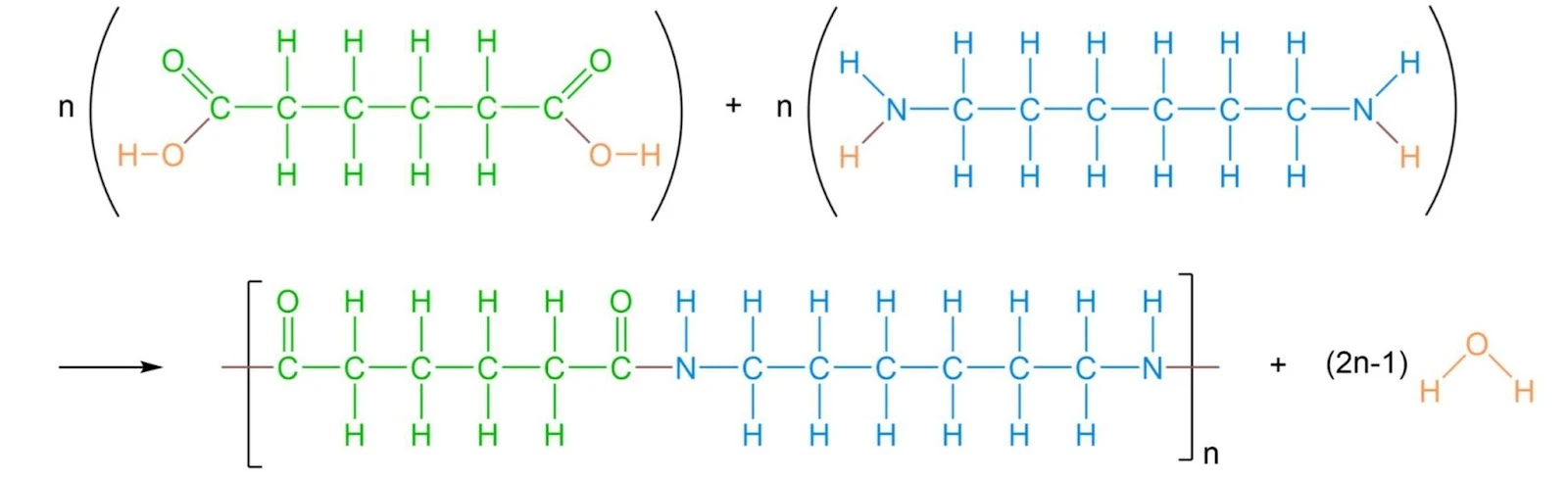
When writing the structural equations for condensation polymerisation, make sure to be careful when writing how many water molecules are formed! This is as condensation polymers can have more than one monomer and this small loss of a molecule only occurs for condensation polymerisation, not addition.
HSC Chemistry Module 8: Applying Chemical Ideas
Ion Testing
Ion testing refers to the processes and tests that can systematically be taken such that the presence of particular ions in an unknown solution can be identified. The syllabus provides a certain selection of cations and anions that you should distinguish between through primarily three different kinds of reactions and processes:
- Flame Tests
Metal cations show unique colours when exposed to an open flame. For example, if a solution containing barium ions was sprayed onto a flame, it would turn apple green and thus allow us to confirm the presence of barium ions.
- Precipitation Reactions
We can add certain solutions and observe for the formation of a precipitate and its colour. Applying our knowledge on solubility rules, our observations can lead us to conclude the presence or absence of certain ions. For instance, we can confirm the presence of iron(II) ions by adding 2 drops of 1M sodium hydroxide and observing for the formation of a green precipitate, iron(II) hydroxide.
- Complexation Reactions
Similarly to precipitates, certain complexes can show unique colours, and is thus another tool that we can use in our ion identification process.
By noting expected observations and the tools above, it is highly recommended that you put together your own ion testing flow chart that works for you. By using a visual medium, it can be much easier to organise the information and memorise observations!
Chemical Synthesis and Design
When evaluating the factors involved in chemical synthesis and design, the most common and effective reaction to look at is the Haber Process. The Haber Process is the industrial process in producing ammonia as follows:
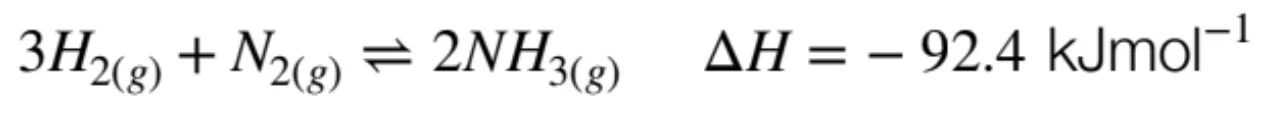
You should be familiar with justifying the economic viability and reaction efficiency of the Haber Process, by applying Le Chatelier’s Principle and Collision Theory to discuss the compromise of reaction conditions (temperature and pressure) to maximise both yield and reaction rate, the catalyst used as well as the sourcing of reagents and other factors such as safety, transport and more!